Cartridge Information
How are the 3 mL cartridges sterilized?
For both of our 3 mL cartridge models, individual components are steam sterilized prior to assembly. Components are then mechanically assembled in a controlled sterile environment, individually sealed in Tyvek steam sterilization pouches, and autoclaved for final steam sterilization. Each pouch includes a visual steam indicator confirming proper exposure to sterilization conditions.
Are the cartridges individually sterilized?
Yes. Each cartridge is individually packaged and undergoes a final steam sterilization cycle after assembly.
Who performs the sterilization process?
Sterilization is performed as part of the manufacturer’s controlled production process prior to shipment.
What type of packaging is used?
Each cartridge is individually sealed in a Tyvek steam sterilization pouch. Tyvek is widely used for steam sterilization due to its durability, breathability, and ability to maintain package integrity following autoclave processing.
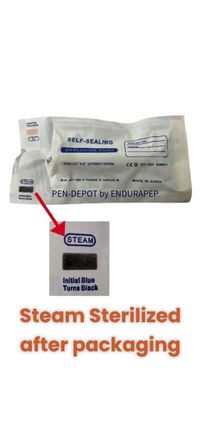
How can I tell the product has been steam sterilized?
Each sealed pouch includes a steam-activated visual indicator. This indicator changes color from Blue to Black when appropriate temperature and steam exposure conditions have been met during the autoclave cycle, providing clear visual confirmation of processing.
Do you provide sterilization certificates, validation logs, or testing reports?
No. We do not provide individual sterilization certificates, batch records, biological indicator results, or testing reports.
Verification is provided solely by the visual steam indicator on the sealed pouch.
Our products are not marketed or classified as regulated medical devices. The sterilization process described reflects quality-controlled handling appropriate for the product’s intended classification and use.
Why don’t you offer additional sterilization documentation?
Individual certification and validation reporting is typically reserved for regulated medical or surgical devices. For our research product category, visual indicator verification on sealed sterilization pouches is the accepted standard.
How do I know if this product is right for my needs?
Customers are encouraged to assess suitability based on their own research requirements and intended use prior to purchase.
If additional documentation or regulatory validation is required for a specific research use case, this product may not be the appropriate fit.
